Temperature and Radiation
Correlation Between Temperature and Radiation
In the 19th century, Lord Kelvin created the Kelvin temperature scale to measure very low temperatures. Because zero Kelvin is considered to be the lowest temperature possible, it is described as absolute zero. There are no negative numbers in the Kelvin scale.

Image Credit: Wikipedia

Image Credit: Microsoft Clip Art
When an object is hot enough, you can see the radiation it emits as visible light. For example, when a stovetop burner reaches 1,000 Kelvin (K) — 726° Celsius (C) or 1,340° Fahrenheit (F) — it will glow red. All objects actually emit radiation if their temperature is greater than absolute zero. Absolute zero is equal to zero Kelvin, which is equal to -273°C or -460°F.
Both the sun and Earth's surface behave as blackbodies. An object that absorbs and emits all possible radiation at 100 percent efficiency is called a blackbody. For this reason, the following two laws (Stefan-Boltzmann and Wein's laws) can be used to explain the correlation between temperature and radiation for the sun and Earth.
The Stefan-Boltzmann law, a fundamental law of physics, explains the relationship between an object's temperature and the amount of radiation that it emits. This law (expressed mathematically as E = σT4) states that all objects with temperatures above absolute zero (0K or -273°C or -459°F) emit radiation at a rate proportional to the fourth power of their absolute temperature.
E = σT4
Stefan-Boltzmann Law
E represents the maximum rate of radiation (often referred to as energy flux) emitted by each square meter of the object's surface. The Greek letter “σ” (sigma) represents the Stefan-Boltzmann constant (5.67 x 10-8W/m2K4); and T is the object's surface temperature in Kelvin. The W refers to watt, which is the unit used to express power (expressed in joules per second).
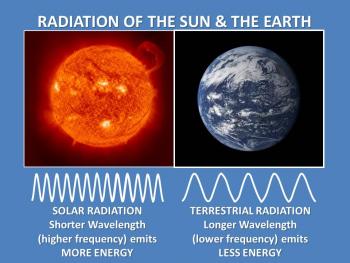
Using the Stefan-Boltzmann law, let's compare the sun's average surface temperature of approximately 6,000K (5,727°C or 10,340°F) with Earth's average surface temperature of just 288K (15°C or 59°F). Consistent with the Stefan-Boltzmann law, the sun emits more radiation than Earth.
Wien's law, another law of physics, (expressed mathematically as λ max = constant/T) explains the relationship between the object's temperature and the wavelength it emits.
λ max = constant/T
Wien's Law
The wavelength at which maximum radiation is emitted is expressed by the Greek letter “ λ ” (lambda). T is the object's temperature in Kelvin, and the constant is 2,897 μm (micrometers). The higher the object's temperature, the faster the molecules will vibrate and the shorter the wavelength will be.
Consequently, Wein's law explains why the hot sun emits radiation at relatively shorter wavelengths, with the maximum emission in the visible region of the spectrum, whereas the relatively cool Earth emits almost all of its energy at longer wavelengths in the infrared region of the spectrum. For this reason, solar radiation is often referred to as shortwave radiation, and terrestrial radiation as longwave radiation.
Heat Transfer in Earth's Atmosphere
Understanding the basic mechanism of heat transfer within Earth's atmosphere and between its surfaces (land and water) and the atmosphere will help you learn how Earth's energy balance works to regulate our climate. To begin, let's review the difference between heat and temperature.
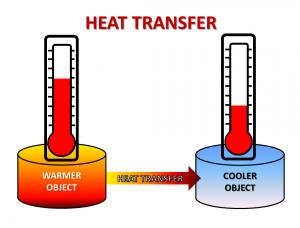
Heat is energy in the process of being transferred from one substance (or object) to another. This process occurs when there is a temperature difference between the two substances. Heat is always transferred from a warmer object to a cooler one. Temperature is a measurement of the average speed of the atoms and molecules that make up a substance.
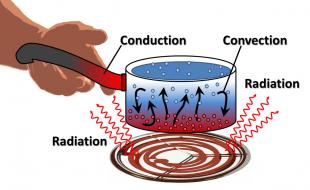
In the previous section, you learned about radiation. Radiation is the mechanism by which solar energy reaches Earth. When Earth absorbs the sun's energy (most of which arrives in the form of visible light), the energy changes into heat. Some of that energy, in turn, is then radiated away from Earth's surface. Because the atmosphere is heated from below, the temperature in the troposphere decreases with height. Heat energy is also spread throughout Earth's atmosphere through conduction and convection.
Conduction is the direct spread of heat from a warmer substance (in this case, land or water) to a cooler substance (the atmosphere). The heat energy transfers when molecules collide with one another. Therefore, conduction, as a heat transfer mechanism, occurs at Earth's surface where the air is in direct contact with the surface.
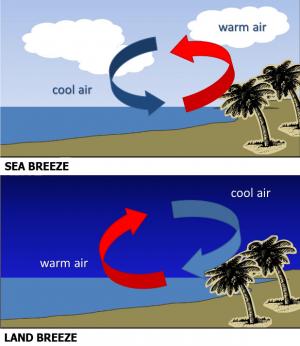
Heat is transferred vertically in the troposphere by convection. Convection is the spread of heat in a fluid, defined as a gas or liquid in which atoms and molecules are moving relatively freely. Consequently, convection can occur in the atmosphere or in bodies of water. Convection currents form when there is unequal heating of the atmosphere or water. As air or water warms, it expands and becomes less dense than the air or water above, and it rises. As air or water cools, its density increases and it sinks.
Conduction and convection work together to transfer heat. We can sense the resulting change in temperature, so these heat transfer mechanisms are known as sensible heating.
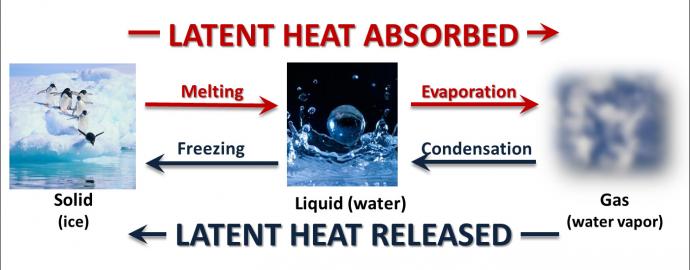
Another type of important heat transfer process affecting the climate system occurs when water undergoes a change in phase. In other words, it changes from a liquid, solid, or gas (water vapor) into a different form or phase (melting ice is an example of a phase change). The reason that heat is transferred as water changes phase is due to the hydrogen bond between molecules of water. Extra energy is needed to break this strong bond and change water from one phase to another.
When water changes phase, heat is exchanged between the water and its surroundings — the water either absorbs or releases heat depending on the phase change. This type of heat is called latent heat, because that heat is stored or hidden until the phase change occurs.
Heat is absorbed when water changes from a liquid to a gas (water vapor). This energy that is absorbed gives the molecules the extra motion that is needed to escape the surface of the liquid to become a gas. This process is known as evaporation, and the absorption of heat is called the latent heat of evaporation (or latent heat of vaporization). When the solid phase (ice) changes to a liquid, melting occurs and heat is also absorbed.
Heat is released when water changes from a gas (water vapor) to a liquid. This happens as warm and humid air rises through the atmosphere into cooler temperatures. Cooler air cannot hold as much moisture, so the water vapor condenses. The latent or hidden heat is then released, which is why this process is known as the latent heat of condensation. Heat is also released when water's liquid phase changes to a solid phase (or freezes).