The Greenhouse Effect
 Joseph Fourier. Image Credit: New World Encyclopedia
Joseph Fourier. Image Credit: New World Encyclopedia
In 1827, Joseph Fourier, a French mathematician and physicist, wondered why Earth's average temperature is approximately 15°C (59°F). He reasoned that there must be some type of balance between the incoming energy and the outgoing energy to maintain this fairly constant temperature. His calculations indicated that Earth should actually be much colder (-18°C or 0°F).
To have an average temperature of 15°C (59°F), Fourier knew that there had to be another process occurring in the atmosphere –– something similar to the way a greenhouse retains heat. A greenhouse's glass enclosure allows visible light to enter and be absorbed by the plants and soil. The plants and soil then emit the absorbed heat energy as infrared radiation. The glass of the greenhouse then absorbs that infrared radiation, emitting some of it back into the greenhouse and thus keeping the greenhouse warm even when the temperature outside is lower.
Because the two processes are similar, the name “greenhouse effect” was coined to describe Fourier's explanation. However, part of a greenhouse's warmth results from the physical barrier of the glass, which prevents the warmer air from flowing outward. So despite the fact that the atmospheric greenhouse effect has some processes in common with an actual greenhouse, the overall mechanisms driving the greenhouse effect are different and more complex.
Greenhouse Gases
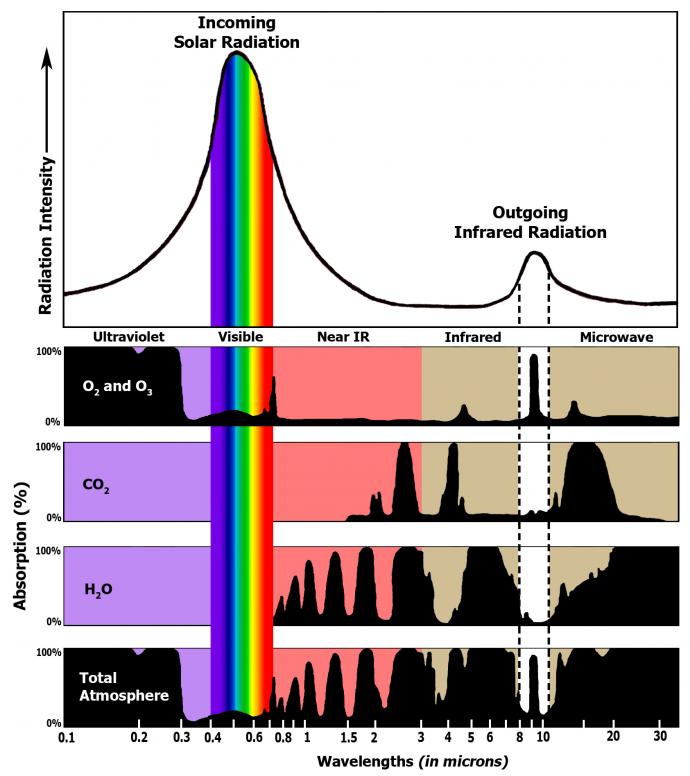
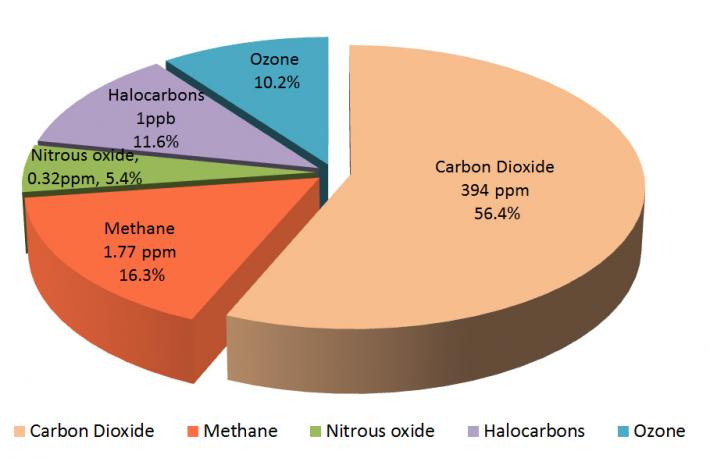
You have already learned that Earth's atmosphere is composed primarily of nitrogen and oxygen. These gases are transparent to incoming solar radiation. They are also transparent to outgoing infrared radiation, which means that they do not absorb or emit solar or infrared radiation. However, there are other gases in Earth's atmosphere that do absorb infrared radiation. These gases are known as greenhouse gases. Below are the most important greenhouse gases that influence Earth's climate system.
Water vapor (H2O) is the strongest greenhouse gas, and the concentration of this gas is largely controlled by the temperature of the atmosphere. As air becomes warmer, it can hold more moisture or water vapor. When the air becomes saturated (or holds as much moisture as the air can at that temperature), the excess moisture will condense into cloud droplets. And if these droplets are large enough, they will fall as precipitation.
Carbon dioxide (CO2) is also an important greenhouse gas. It has a long lifetime in Earth's atmosphere. Carbon dioxide strongly absorbs energy with a wavelength of 15 μm (micrometers). This makes carbon dioxide a good absorber of wavelengths falling in the infrared radiation region of the spectrum.
Carbon dioxide constantly moves into and out of the atmosphere through four major processes: photosynthesis, respiration, organic decomposition or decay, and combustion or the burning of organic material. You will learn more about carbon dioxide and the carbon cycle in Module 4.
Methane (CH4) is 30 times stronger than carbon dioxide as an absorber of infrared radiation. Methane, however, is present in smaller concentrations than carbon dioxide, so its net contribution to the greenhouse effect is not as large. Methane is also relatively short-lived (lasting approximately 8 years) in the atmosphere. Methane is produced when bacteria decompose organic plant and animal matter in such places as wetlands (e.g., marshes, mudflats, flooded rice fields), sewage treatment plants, landfills, and the guts of cattle and termites. Scientists are concerned about the concentration of methane increasing in regions where the Arctic and alpine permafrost is thawing and releasing methane as it warms.
Halocarbons are composed of carbon, chlorine, fluorine, and hydrogen. They include chlorofluorocarbons (CFCs), which are man-made gases commonly used in refrigerators and air conditioners. Concentrations of CFC gases in the atmosphere are the highest of any of the halocarbons, and they can absorb more infrared radiation than any other greenhouse gas. The impact of 1 molecule of a CFC gas is equivalent to 10,000 molecules of carbon dioxide.
Nitrous oxide (N2O), a relatively long-lived gas, has increased in atmospheric concentration due mainly to agriculture. Nitrate (NO3-) and ammonia (NH4+) are used as fertilizers. Bacteria convert a small amount of this nitrate and ammonia into the form of nitrous oxide. Internal combustion engines also produce nitrous oxide.
Ozone (O3) is also a relatively minor greenhouse gas because it is found in relatively low concentrations in the troposphere (the lowest layer of the atmosphere). In the troposphere, it is produced by a combination of pollutants — mostly hydrocarbons and nitrogen oxide compounds.
 John Tyndall. - Image Credit: Wikipedia
John Tyndall. - Image Credit: Wikipedia
In the 1860s, John Tyndall, an Irish scientist who was fascinated by the growth and formation of glaciers, wanted to test his ideas explaining how Earth maintained a fairly constant temperature. He began a series of experiments to measure the amount of radiant heat (infrared radiation) that certain gases could absorb and transmit. Tyndall found that water vapor and carbon dioxide were good absorbers and emitters of infrared radiation.
The relative importance of a greenhouse gas depends on its abundance in Earth's atmosphere and how much the gas can absorb specific wavelengths of energy.
An effective absorber of infrared radiation has a broader absorption profile, which means that it can absorb a wider spectrum of wavelengths. Water vapor and carbon dioxide can absorb radiation wavelengths in the range of 4 μm to 80 μm, except those between 8 μm and 12 μm. Ozone can absorb wavelengths between 9 μm and 10 μm, but as you have learned, it is found in low concentrations. The sun's ultraviolet wavelengths are strongly absorbed by ozone in the stratosphere.
How the Greenhouse Effect Works
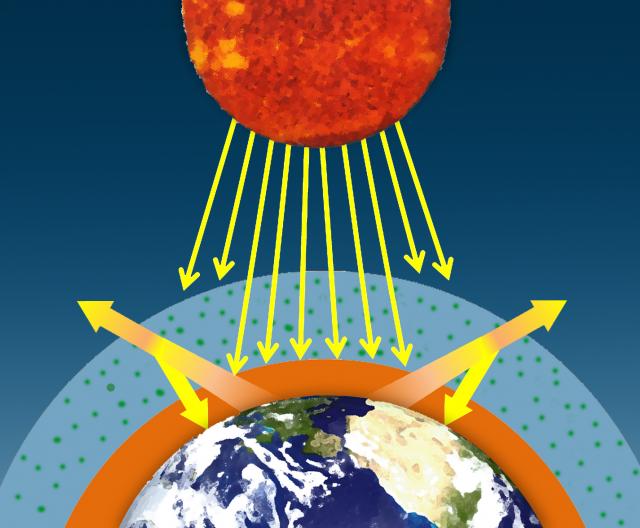
The sun's visible wavelengths of radiation pass easily through the atmosphere and reach Earth. Approximately 51% of this sunlight is absorbed at Earth's surface by the land, water, and vegetation. Some of this energy is emitted back from the Earth's surface in the form of infrared radiation.
Water vapor, carbon dioxide, methane, and other trace gases in Earth's atmosphere absorb the longer wavelengths of outgoing infrared radiation from Earth's surface. These gases then emit the infrared radiation in all directions, both outward toward space and downward toward Earth. This process creates a second source of radiation to warm to surface – visible radiation from the sun and infrared radiation from the atmosphere – which causes Earth to be warmer than it otherwise would be. This process is known as the natural greenhouse effect and keeps Earth's average global temperature at approximately 15°C (59°F).
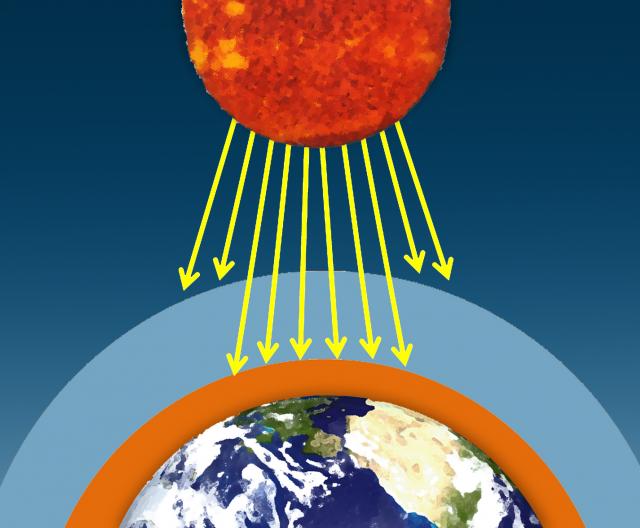
I. The sun's visible wavelengths of radiation pass easily through the atmosphere and reach Earth. Approximately 51% of this sunlight is absorbed at Earth's surface by the land, water, and vegetation.
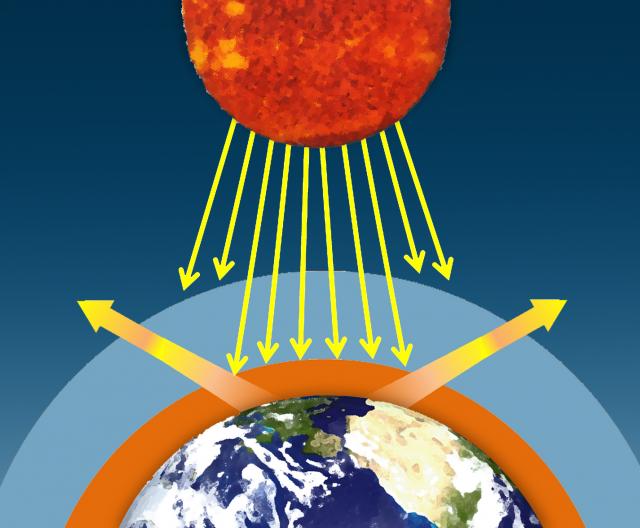
II. Some of this energy is emitted from Earth's surface back into space in the form of infrared radiation.
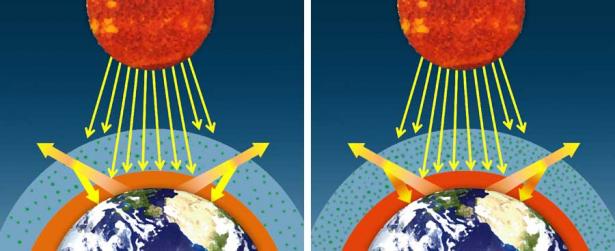
If the concentration of greenhouse gases increases, then more infrared radiation will be absorbed and emitted back toward Earth's surface, creating an enhanced or amplified greenhouse effect.
When averaged over the course of a year, the amount of incoming solar radiation received from the sun has balanced the amount of outgoing energy emitted from Earth. This equilibrium is called Earth's energy or radiation balance. Relatively small changes in the amounts of greenhouse gases in Earth's atmosphere can greatly alter that balance between incoming and outgoing radiation. Earth then warms or cools in order to restore the radiative balance at the top of the atmosphere.