Water's Influence on Temperature
One unique property of water is its high heat capacity – the highest of all liquids other than liquid ammonia. This property is due to the hydrogen bond between water molecules. The following explanation about water molecules will help you understand why coastal areas tend to have more moderate temperatures.
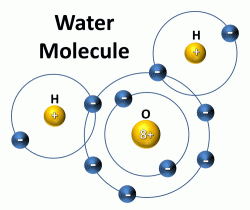
A water molecule consists of one oxygen (O) atom bonded to two hydrogen (H) atoms. The “8+” refers to the atomic number of oxygen, which is also the number of protons in the nucleus and number of electrons in the energy levels outside the nucleus. For the oxygen atom, 2 electrons are in the first energy level and the remaining 6 electrons are in the next or second energy level. Hydrogen has an atomic number of 1, which means that hydrogen has one proton in the nucleus and one electron in the lowest energy level outside the nucleus. The second energy level can hold 8 electrons, so each hydrogen atom shares its 1 electron with the oxygen atom, completing the second energy level and making a water molecule.
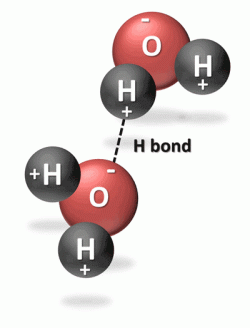
The bonding between the oxygen atom and each hydrogen atom is known as covalent bonding because they share electrons to make a very stable water molecule. The two hydrogen atoms are bonded to the oxygen atom at a 105° angle. This geometry of the water molecule causes it to have positively and negatively changed ends, known as polarity. Water is referred to a polar or dipolar molecule. The large nucleus of the oxygen atom attracts the shared electrons causing this side of the water molecule to be negatively charged while the hydrogen side is positively charged. This polarity allows water to bond easily with adjacent water molecules. The hydrogen bond is the bond between two water molecules.
Water is a liquid rather than gas (or water vapor) at room temperature because of the strong hydrogen bond between the molecules of water. (This strong bond causes water to resist molecular motion and remain a liquid at room temperature.) This means that it takes more energy or heat to increase water’s temperature than it does for most other substances. Specific heat is the amount of heat energy it takes to raise or lower the temperature of 1 gram of a substance by 1°Celsius. The specific heat of liquid water is 1 calorie per gram per 1 degree C (cal/g/°C). The specific heat of water is greater than that of dry soil, therefore water both absorbs and releases heat more slowly than land.
Water also is fluid, allowing the heat to be mixed to greater depth than on land. The heat capacity is the product of the specific heat and the mass (in g) of the material. Oceans have a greater heat capacity than land because the specific heat of water is greater than that of dry soil and because a mixing of the upper ocean results in a much larger mass of water being heated than land. This causes land areas to heat more rapidly and to higher temperatures and also cool more rapidly and to lower temperatures, compared to oceans.
The high heat capacity of water also explains why the temperatures of land near a body of water are more moderate. The high heat capacity of water keeps its temperature within a relatively narrow range, causing nearby coastal areas to also have a narrow daily and seasonal temperature range. In contrast, areas with similar weather conditions that are farther from the coast tend to have a much wider range of seasonal and daily temperatures. To summarize, large bodies of water tend to moderate the temperature of nearby land due to the high heat capacity of water. This high heat capacity results from both the higher specific heat of water and the mixing of heat throughout a greater depth over oceans.