The Oxygen and Hydrogen Isotope Ratio
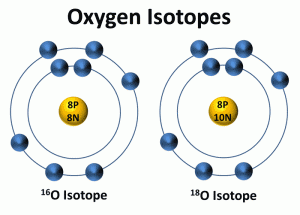
The oxygen isotope ratio is the first way used to determine past temperatures from the ice cores. Isotopes are atoms of the same element that have a different number of neutrons. All isotopes of an element have the same number of protons and electrons but a different number of neutrons in the nucleus. Because isotopes have a different number of neutrons, they have different mass numbers. Oxygen's most common isotope has a mass number of 16 and is written as 16O. Most of the oxygen in water molecules is composed of 8 protons and 8 neutrons in its nucleus, giving it a mass number (the number of protons and neutrons in an element or isotope) of 16. About one out of every 1,000 oxygen atoms contains 2 additional neutrons and is written as 18O.
Depending on the climate, the two types of oxygen (16O and 18O) vary in water. Scientists compare the ratio of the heavy (18O) and light (16O) isotopes in ice cores, sediments, or fossils to reconstruct past climates. They compare this ratio to a standard ratio of oxygen isotopes found in ocean water at a depth of 200 to 500 meters. The ratio of the heavy to light oxygen isotopes is influenced mainly by the processes involved in the water or hydrologic cycle.
More evaporation occurs in warmer regions of the ocean, and water containing the lighter 16O isotope evaporates more quickly than water containing the heavier 18O. Water vapor containing the heavier 18O, however, will condense and precipitate more quickly than water vapor containing the lighter 16O. As water evaporates in warmer regions, it is moved with air by convection toward the polar regions.
Ocean-floor sediments can also be used to determine past climate. They reflect the oxygen isotope of the ocean water, because the oxygen in the calcium carbonate shells that are deposited on the ocean floor records the oxygen isotope variations in the ocean at the time of formation.
The table explains how the oxygen isotope ratio can be used to reconstruct the type of past climate. The table explains the oxygen isotope ratio for ice cores and ocean water/ocean floor sediments during a colder climate or glacial period.
| Colder Climates | ||
|---|---|---|
| Oxygen Isotopes Ratio | Explanation | |
| Ice Core | Ice cores contain more 16O than ocean water, so ice cores have a lower 18O/ 16O ratio than ocean water or ocean-floor sediments. | Water containing the lighter isotope 16O evaporates more readily than 18O in the warmer subtropical regions. As this water vapor (which is enriched with 16O) moves toward the poles, the heavier 18O condenses and precipitates out first at lower latitudes, leaving progressively more 16O in the water vapor reaching the poles. The water vapor that reaches the polar regions precipitates as snow, eventually becoming ice. |
| Ocean Water/Sediments | Ocean water and ocean-floor sediments contain more 18O than ice cores, so the ocean water and sediments have a higher 18O/ 16O ratio than ice cores. | When the ocean is colder, it takes more energy to evaporate the heavier isotope, 18O, than it does to evaporate the lighter isotope, 16O. The water vapor with 18O condenses and precipitates out first at lower latitudes. This causes the oceans to have more 18O. |
In a warmer climate, ocean water would contain more 16O because as ice sheets melt, the water with 16O is returned to the ocean.
The Deuterium to Hydrogen Ratio
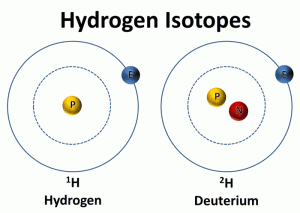
The second way to determine past temperatures is by calculating the deuterium to hydrogen ratio in the ice core samples. The water molecule contains two different isotopes of hydrogen (1H and 2H). 1H contains one proton and no neutrons and 2H, known as deuterium or D, contains one proton and one neutron. The ratio of deuterium to hydrogen in the ice core is compared to the ratio of deuterium to hydrogen in standard mean ocean water. The ice cores contain slightly less of the heavier isotopes of oxygen (18O) and deuterium (2H).